We use cookies to understand how you use our site and to improve your experience. This includes personalizing content and advertising. To learn more, click here. By continuing to use our site, you accept our use of cookies, revised Privacy Policy and Terms of Service.
You are being directed to ZacksTrade, a division of LBMZ Securities and licensed broker-dealer. ZacksTrade and Zacks.com are separate companies. The web link between the two companies is not a solicitation or offer to invest in a particular security or type of security. ZacksTrade does not endorse or adopt any particular investment strategy, any analyst opinion/rating/report or any approach to evaluating individual securities.
If you wish to go to ZacksTrade, click OK. If you do not, click Cancel.
Amicus (FOLD) Issues Preliminary Revenue Results for FY23
Read MoreHide Full Article
Amicus Therapeutics, Inc. (FOLD - Free Report) announced preliminary results for the fourth quarter and full-year 2023. The company also provided business updates and a strategic outlook for 2024.
Q4 and 2023 Preliminary Results
Preliminary total revenues were around $399.4 million for full-year 2023, reflecting an increase of 20% at constant exchange rates (CER). For the fourth quarter, preliminary total revenues were approximately $115.1 million.
For the full year, Galafold (migalastat) net product sales were approximately $387.8 million, increasing 17% year over year at CER. Galafold net sales were around $106.6 million in the fourth quarter.
Newly approved products, Pombiliti (cipaglucosidase alfa) + Opfolda (miglustat), generated sales of roughly $11.6 million for full-year 2023. For the fourth quarter of 2023, Pombiliti + Opfolda net product sales were approximately $8.5 million.
Shares of Amicus have rallied 19.6% in the past year against the industry’s 11.4% decline. Image Source: Zacks Investment Research
Amicus’ lead drug, Galafold, is the first and only approved oral medicine for patients living with Fabry disease. The drug has shown solid uptake since its launch. Galafold is currently approved in several countries, including the United States, the European Union, the United Kingdom and Japan.
In September 2023, the FDA approved Pombiliti + Opfolda 65mg capsules, a two-component therapy for treating late-onset Pompe disease (LOPD) in adults who weigh more than 40 kg and are not improving on their current enzyme replacement therapy.
Earlier in 2023, Pombiliti + Opfolda was approved in Europe, including the United Kingdom, for treating LOPD in adults. Global launches of Pombiliti and Opfolda are currently underway, including in the United States, EU and United Kingdom.
2024 Key Updates
Amicus remains focused on delivering Galafold revenue growth of 11-16% at CER in 2024. The company is also looking to ensure the successful global launches of Pombiliti and Opfolda in Pompe disease.
This apart, FOLD remains focused on advancing its ongoing label expansion studies in two rare diseases such as Fabry and Pompe disease.
The company targets to achieve full-year profitability on a non-GAAP basis in 2024.
In the past 60 days, estimates for Sarepta’s 2024 earnings per share have improved from 46 cents to $1.72. In the past year, shares of SRPT have plunged 17.2%.
The earnings of Sarepta beat estimates in each of the last four quarters. SRPT delivered a four-quarter earnings surprise of 48.67%, on average.
In the past 60 days, estimates for Entrada Therapeutics’ 2024 loss per share have narrowed from $2.35 to $2.04. In the past year, shares of TRDA have inched up 0.1%.
The earnings of Entrada Therapeutics beat estimates in three of the last four quarters while missing the same on the remaining occasion. TRDA delivered a four-quarter average earnings surprise of 70.68%.
In the past 60 days, estimates for Puma Biotechnology’s 2024 earnings per share have improved from 62 cents to 69 cents. In the past year, shares of PBYI have risen 1.7%.
The earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
See More Zacks Research for These Tickers
Normally $25 each - click below to receive one report FREE:
Image: Bigstock
Amicus (FOLD) Issues Preliminary Revenue Results for FY23
Amicus Therapeutics, Inc. (FOLD - Free Report) announced preliminary results for the fourth quarter and full-year 2023. The company also provided business updates and a strategic outlook for 2024.
Q4 and 2023 Preliminary Results
Preliminary total revenues were around $399.4 million for full-year 2023, reflecting an increase of 20% at constant exchange rates (CER). For the fourth quarter, preliminary total revenues were approximately $115.1 million.
For the full year, Galafold (migalastat) net product sales were approximately $387.8 million, increasing 17% year over year at CER. Galafold net sales were around $106.6 million in the fourth quarter.
Newly approved products, Pombiliti (cipaglucosidase alfa) + Opfolda (miglustat), generated sales of roughly $11.6 million for full-year 2023. For the fourth quarter of 2023, Pombiliti + Opfolda net product sales were approximately $8.5 million.
Shares of Amicus have rallied 19.6% in the past year against the industry’s 11.4% decline.
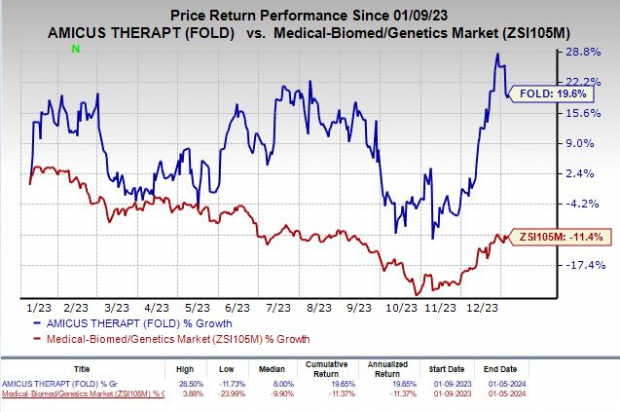 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
Amicus’ lead drug, Galafold, is the first and only approved oral medicine for patients living with Fabry disease. The drug has shown solid uptake since its launch. Galafold is currently approved in several countries, including the United States, the European Union, the United Kingdom and Japan.
In September 2023, the FDA approved Pombiliti + Opfolda 65mg capsules, a two-component therapy for treating late-onset Pompe disease (LOPD) in adults who weigh more than 40 kg and are not improving on their current enzyme replacement therapy.
Earlier in 2023, Pombiliti + Opfolda was approved in Europe, including the United Kingdom, for treating LOPD in adults. Global launches of Pombiliti and Opfolda are currently underway, including in the United States, EU and United Kingdom.
2024 Key Updates
Amicus remains focused on delivering Galafold revenue growth of 11-16% at CER in 2024. The company is also looking to ensure the successful global launches of Pombiliti and Opfolda in Pompe disease.
This apart, FOLD remains focused on advancing its ongoing label expansion studies in two rare diseases such as Fabry and Pompe disease.
The company targets to achieve full-year profitability on a non-GAAP basis in 2024.
Zacks Rank & Stocks to Consider
Amicus currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Sarepta Therapeutics, Inc. (SRPT - Free Report) Entrada Therapeutics, Inc. (TRDA - Free Report) and Puma Biotechnology, Inc. (PBYI - Free Report) , each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Sarepta’s 2024 earnings per share have improved from 46 cents to $1.72. In the past year, shares of SRPT have plunged 17.2%.
The earnings of Sarepta beat estimates in each of the last four quarters. SRPT delivered a four-quarter earnings surprise of 48.67%, on average.
In the past 60 days, estimates for Entrada Therapeutics’ 2024 loss per share have narrowed from $2.35 to $2.04. In the past year, shares of TRDA have inched up 0.1%.
The earnings of Entrada Therapeutics beat estimates in three of the last four quarters while missing the same on the remaining occasion. TRDA delivered a four-quarter average earnings surprise of 70.68%.
In the past 60 days, estimates for Puma Biotechnology’s 2024 earnings per share have improved from 62 cents to 69 cents. In the past year, shares of PBYI have risen 1.7%.
The earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.